Monika Cechova
Biography
I am interested in the most complex parts of the human genome. I believe complete, Telomere-To-Telomere assemblies are the future of genomics that is happening now.
Interests
- Long reads and complete T2T genomes
- Y chromosome, centromeres, telomeres, acrocentric chromosomes
- Satellite Biology and Heterochromatin
- Aneuploidies
Education
-
PhD Major in Biology, Minor in Statistics, 2020
Penn State, USA
-
MS in Bioinformatics, 2013
Masaryk University, Brno
-
BS in Applied Informatics, 2011
Masaryk University, Brno
Skills
R
Statistics
Python
Nanopore
6 years
PacBio
8 years
Illumina
12 years
Experience
Assistant Professor
Faculty of Informatics, Masaryk University, Brno
- Complex parts of human genomes: Y chromosome, acrocentric chromosomes, centromeres, telomeres
- Aneuploidies, machine learning for genomics
Postdoc
Department of Biomolecular Engineering, University of California, Santa Cruz
- T2T and HPRC consortia
- Gaining new understanding of the satellite DNA
Postdoc
Faculty of Informatics, Masaryk University
- Developing new algorithms, tools, and methods for bioinformatics
- Gaining new understanding of the repetitive DNA
Postdoc
Institute of Animal Physiology and Genetics CAS, v. v. i. Central European Institute of Technology; Department of Genetics and Reproduction, Veterinary Research Institute
- Early Embryonic Development
- Spindle Assembly Checkpoint
Graduate Student
Penn State
- Studied driving forces of Y chromosome evolution in great apes (Cechova, Vegesna et al. 2020)
- Explored evolution of heterochromatin in great apes (Cechova et al., 2019)
- Characterized genome-wide effects of non-B DNA on polymerization speed and error rate (Guiblet et al., 2018)
- Developed algorithms for the Y chromosome assembly (Rangavittal et al., 2018)
- Characterized genes, repeats and palindromes on gorilla Y chromosome (Tomaszkiewicz, Rangavittal, Cechova et al. 2016)
- Developed hybrid genome assembly algorithms for combining short and long reads (Tomaszkiewicz, Rangavittal, Cechova et al. 2016)
Bioinformatician
Institute of Biophysics, Academy of Sciences of the Czech Republic
- Developed pipelines for detection of sex-linked genes from NGS data (Cechova et al., 2015)
- Characterized nupts and numts in 6 plant species, their age, length, distribution, consequences of insertions, etc. (Cechova et al., 2013)
- Explored microsatellites-TEs association and microsatellite periodicity (Kejnovsky et al., 2013)
Awards
The program of support for promising postdoctoral students (PPLZ)
Mohnkern scholarship
Hill-Hill Memorial Fund Fellowship
Troxell Memorial Scholarship in Biology
CBIOS (NIH T32 Predoctoral Training Grant)
Braddock Scholarship
Best Poster Award, Genetics Conference, Lednice
Featured Publications
The complete sequence of a human Y chromosome
The human Y chromosome has been notoriously difficult to sequence and assemble because of its complex repeat structure that includes long palindromes, tandem repeats and segmental duplications1,2,3. As a result, more than half of the Y chromosome is missing from the GRCh38 reference sequence and it remains the last human chromosome to be finished4,5. Here, the Telomere-to-Telomere (T2T) consortium presents the complete 62,460,029-base-pair sequence of a human Y chromosome from the HG002 genome (T2T-Y) that corrects multiple errors in GRCh38-Y and adds over 30 million base pairs of sequence to the reference, showing the complete ampliconic structures of gene families TSPY, DAZ and RBMY; 41 additional protein-coding genes, mostly from the TSPY family; and an alternating pattern of human satellite 1 and 3 blocks in the heterochromatic Yq12 region. We have combined T2T-Y with a previous assembly of the CHM13 genome4 and mapped available population variation, clinical variants and functional genomics data to produce a complete and comprehensive reference sequence for all 24 human chromosomes.
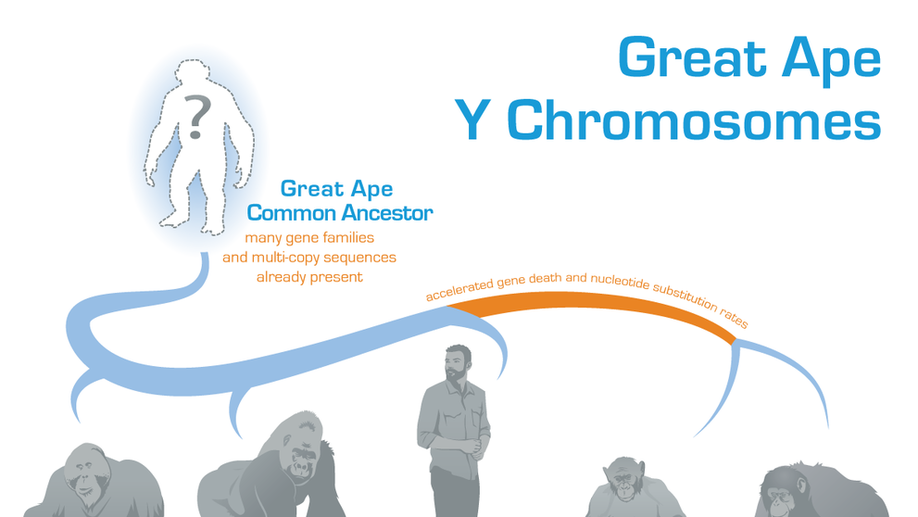
Dynamic evolution of great ape Y chromosomes
The male-specific Y chromosome harbors genes important for sperm production. Because Y is repetitive, its DNA sequence was deciphered for only a few species, and its evolution remains elusive. Here we compared the Y chromosomes of great apes (human, chimpanzee, bonobo, gorilla, and orangutan) and found that many of their repetitive sequences and multicopy genes were likely already present in their common ancestor. Y repeats had increased intrachromosomal contacts, which might facilitate preservation of genes and gene regulatory elements. Chimpanzee and bonobo, experiencing high sperm competition, underwent many DNA changes and gene losses on the Y. Our research is significant for understanding the role of the Y chromosome in reproduction of nonhuman great apes, all of which are endangered.








